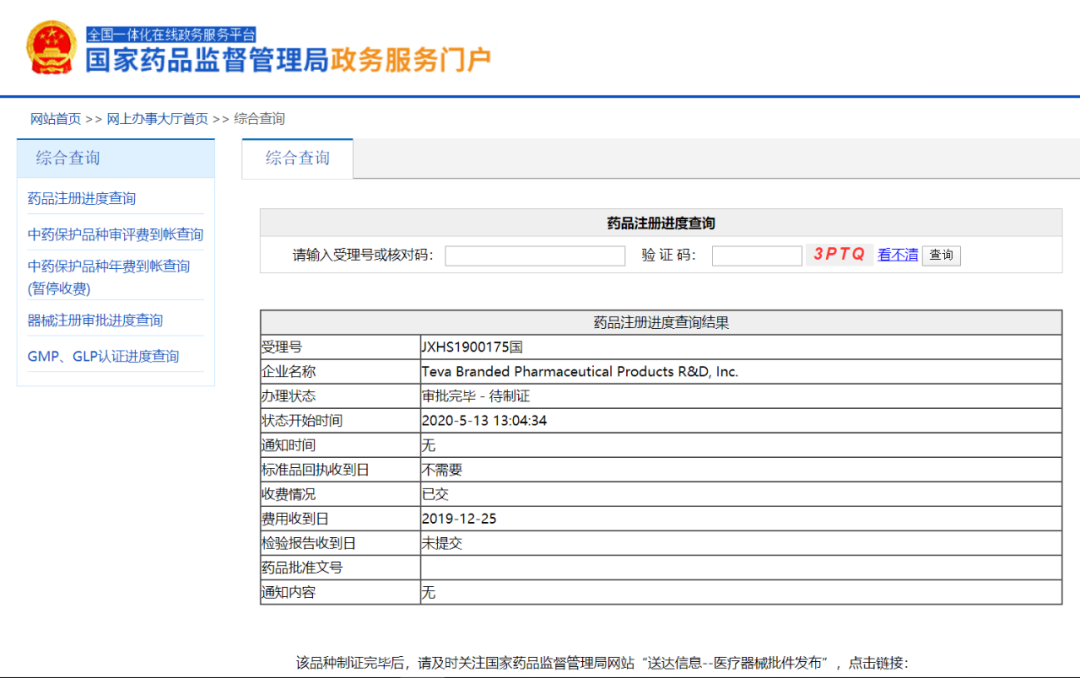
亨廷顿病是一种罕见的致命的神经退化性疾病,亚洲每十万人中大约有0.4人患病[3],平均发病年龄为40岁。舞蹈病( 无意识的、随机和突然的扭动和/或转动 )是这种疾病最为显著的表现之一并发生在大约90%的病人当中。
迟发性运动障碍是一种使人衰弱的运动紊乱,以舌头、嘴唇、脸、躯体和四肢部位的重复且不可控的运动为特征。TD在长期接受抗精神病药治疗的中国的精神分裂症患者当中的患病率为33.7%[4],可能是由某些用于治疗精神健康状况的药物引起的,这意味着使用这些药物的精神分裂症患者中有三分之一可能患有TD。
这种疾病不仅影响患者的治疗依从性,也影响患者的生活质量和他们的社会功能[5]。目前在中国对TD尚无明显有效的疗法。
关于安泰坦® 安泰坦®于2017年4月获得美国FDA批准,是FDA首次批准的氘代产品,也是针对与亨廷顿病有关的舞蹈病的历史上第二个药物[6]。目前,该药已经在美国和中国两个国家获批,在美国的获批适应症包括与亨廷顿病(HD)有关的舞蹈病以及成人迟发性运动障碍(TD)。FDA对安泰坦®的批准代表了HD患者的一个重要的新治疗选择,并强调了对这个服务不足的患者群体更多治疗资源的需求,TD适应症被批准为突破性治疗。我们相信,治疗迟发性运动障碍的医生会欣赏到该疗法的剂量灵活性,以及专注于直接治疗运动障碍而不破坏正在进行的基础疾病治疗的能力[7]。
关于亨廷顿病有关的舞蹈病获批临床试验[8] 安泰坦®批用于治疗与亨廷顿病有关的舞蹈病是基于一项由90例患有明显亨廷顿病相关的舞蹈病患者参与的随机、双盲、安慰剂对照、多中心试验的结果。该研究主要临床评价指标为舞蹈病症状最高总评分(Total Maximal Chorea Score, TMC评分)。研究结果表明安泰坦®:
风信子关爱 风信子亨廷顿舞蹈症关爱中心是一家致力于为亨廷顿舞蹈症患者提供支持的公益组织。机构创始人来自亨廷顿舞蹈症家庭,机构旨在为亨廷顿舞蹈症患者和家属们提供支持和帮助、倡导积极健康的生活态度、改善患者生活质量。 机构的战略目标是结合社会资源,为全国亨廷顿舞蹈症患者群体发声、提供支持和帮助,改善医疗环境,提升患者及其家庭的生活质量。机构使命是在包容的社会环境下,给患者群体带来有尊严、有质量的生活。